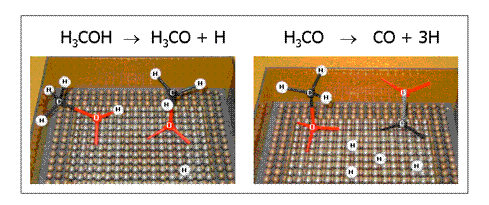
The decomposition of methanol (H3COH) on these metals to hydrogen (H) and carbon monoxide (CO) are studied. This reaction is currently used in methanol powered fuel cells. The methanol is decomposed over Pd catalysts to hydrogen, which is transferred to the fuel cell. The mechanism of the reaction is shown here (Click picture to enlarge).
To gain further insight on the influence of the structure and chemical composition of the nanometer sized active sites the stable intermediates on the surface will be studied using Reflection Absorption Infrared Spectroscopy (RAIRS). This new surface analysis tool is currently being attached to one of our Ultra High Vacuum (UHV) systems. By depositing a metal onto our metallic samples we can prepare defined active sites and tune them to find the best active site for a particular model reaction. This basic knowledge will ultimately lead to the design of new catalysts, which are more active and more selective than the ones currently used in industrial processes.
Recent Publications:
R. Schennach, A. Eichler, K.D. Rendulic, J. Phys. Chem. B 107 (2003) 2552 „Adsorption and desorption of methanol on Pd (111) and on a Pd/V surface alloy“.R. Schennach, G. Krenn, K. D. Rendulic, Vacuum 71 (2003) 89 „The influence of the translational energy of methanol during adsorption on Rh (111) and on a Rh/V surface alloy“.
R. Schennach, G. Krenn, B. Klötzer, K. D. Rendulic, Surf. Sci. 540 (2003) 237 „Adsorption of hydrogen and carbon monoxide on Rh(111)/V surface alloys“.